Description
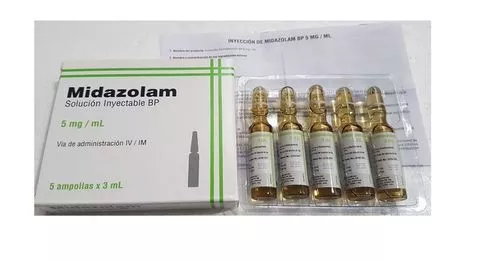
ACTIVE SUBSTANCE CONCENTRATION: 5 mg/mL PHARMACEUTICAL FORM: Solution for injection. ROUTE OF ADMINISTRATION: MI – Intramuscular / IV – Intravenous PRODUCT PRESENTATION: Box x 5 ampoules of type I glass x 3 mL. VALIDITY TIME: 2 years from manufacture. LABORATORY MANUFACTURER: Farbe Firma Pvt LTD. COUNTRY OF ORIGIN: India. EXPORTER: SRS Life Sciences Pte Ltd. STORAGE: Store at a temperature not exceeding 30°C in the original container and packaging. ATC: N05CD08. INDICATIONS AND / OR THERAPEUTIC USE: Sedative, hypnotic. Disorders in the rhythm of sleep and all forms of insomnia, sedation in premediation before interventions or diagnostic procedures. CONTRAINDICATIONS: Contraindicated in patients with known hypersensitivity to benzodiazepines, acute pulmonary failure, severe chronic obstructive pulmonary disease, and acute narrow-angle glaucoma. Administer slowly in elderly or debilitated patients. It has been linked to respiratory arrest.<br />
<br />
*AUTHORIZED PURCHASE FOR PROFESSIONAL USE ONLY - SUBJECTED TO EACH COUNTRY'S REGULAMENTS
- Dormicum
- Sedation
- Anesthetic
- Muscle relaxant
- Covid
- ICU
- intubation
- benzodiazepine
- Anxiolytic
Production Capacity:
Not informed
Delivery Timeframe:
Immediate
Incoterms:
FCA - Free Carrier
Packaging Details:
Not informed
More about
SICMAFARMA
10-50
Employees
0 - 100K
Sales volume (USD)
10%
% Export sales
Year
Established
Business type
- Importer / Trading Company
- Representative / Agent
- Distributor / Wholesaler
Keywords
- medicines
- medical supplies
- antibiotics
- anesthetics
- anti-infectives
- antidiabetes
- oncological
- antineoplastics Ver Mais
Contact and location
-
Gina ********
-
+57 3********
-
Bogotá DC / Bogotá DC | Colombia